How to Overcome the Challenge of Modular Content in Medical Communications
HCPs face intense time pressure while being expected to keep up with an ever-expanding body of medical knowledge. According to an Elsevier study surveying 1,778 physicians, they prioritize:
- Credibility (81%)
- Up-to-date information (78%)
- Ease of searching (60%)
However, traditional content models don’t always meet these expectations. Materials get recreated across formats and geographies, wasting resources and introducing inconsistencies.
Modular content theoretically solves these problems through pre-approved building blocks assembled quickly across channels. It promises everything Medical Affairs teams need: speed, consistency, efficiency.
You’ve most likely heard time and again how modular content is poised to revolutionize Medical Communications. The topic of modular content dominates discussions at MAPS and ISMPP, appears in white papers, and gets positioned as the future of Medical Affairs. Yet actual implementation remains challenging for Medical Affairs teams to achieve.
Modular Infrastructure Challenges in Medical Communications
Modular content sounds perfect in theory. Build content like LEGO blocks. Update your “master deck” once and watch changes automatically cascade through every content item referencing the material. But this vision requires foundational systems many pharmaceutical companies don’t have.
For one thing, modular content depends on standardized metadata and aligned taxonomies. However, the reality is that each function or market uses different naming conventions, storage approaches, and categorization methods. Most repositories aren’t structured libraries—they’re collections of final assets and outdated PDFs that lack consistent tagging or governance.
The automation challenge is equally daunting. Updating content once and having those changes flow automatically requires software that can handle complex approval workflows, regulatory compliance tracking, multi-market version control, and comprehensive audit trails. That software doesn’t exist—at least not in forms that satisfy pharmaceutical industry constraints.
Even if the technology existed, implementation would require coordination across C-level IT, legal, content creators, marketing, and commercial teams. No single team has authority for enterprise-wide transformation. And because every pharmaceutical company functions differently—with content development at country, regional, or global levels—there’s no universal approach.
Despite years of discussion and detailed frameworks, implementing modular content continues to be a challenge. Ambitious goals continue to outpace operational readiness. As a result, the gap keeps widening.
When “Ready to Use” Content Requires Extensive Rework
Even when global teams create structured, scalable materials, local affiliates face significant adaptation requirements such as:
- Label and prescribing differences
- Varying therapy area maturity, local medical priorities
- Country-specific compliance expectations
Affiliates are told content is “ready to use,” yet they still spend considerable time reworking materials to suit their needs. This triggers additional review cycles rather than delivering promised efficiency. Local teams are left feeling as though they’re extending or even duplicating effort rather than benefiting from global work.
Creating Medical Affairs content by traditional means remains slow and redundant. Content is often single-use and costly. Lengthy review cycles create bottlenecks. Even when reused, elements like figures and tables wind up rebuilt from scratch in each market to accommodate language differences, local formatting conventions, and regulatory requirements—wasting time and compromising consistency.
The challenge isn’t the result of a lack of effort. Pharmaceutical operations are so complex that maintaining consistency while allowing necessary local adaptation becomes nearly impossible at scale.
The Alternative: A Centralized Content Creation Framework
Medical Affairs teams still need quality digital materials that are impactful, budget-friendly, and produced rapidly. If modular infrastructure isn’t practically feasible, what does work?
A Centralized Content Creation Framework enables building materials from pre-approved, reusable components without requiring perfect enterprise systems. Start with a strong foundation: one high-value asset like a manuscript, congress data readout, or comprehensive publication. This becomes your “master deck”—a single source of truth to support the next steps in creating a reusable framework:
- Build a component repository. Identify key elements that will appear in multiple outputs: medical statements, graphs, FAQs, mechanism of action descriptions. These aren’t “modules” in the technical sense—they’re governed scientific building blocks with clear sourcing and approval status.
- Secure pre-approval. Run core components through medical and legal review before creating derivative materials. Approve the building blocks themselves, not just finished products. Create approved templates for common formats.
This enables strategic reuse. Teams work from pre-approved components rather than starting from scratch. This isn’t automated drag-and-drop—that software doesn’t exist. It’s a repository for strategic, governed copy-paste from approved sources.
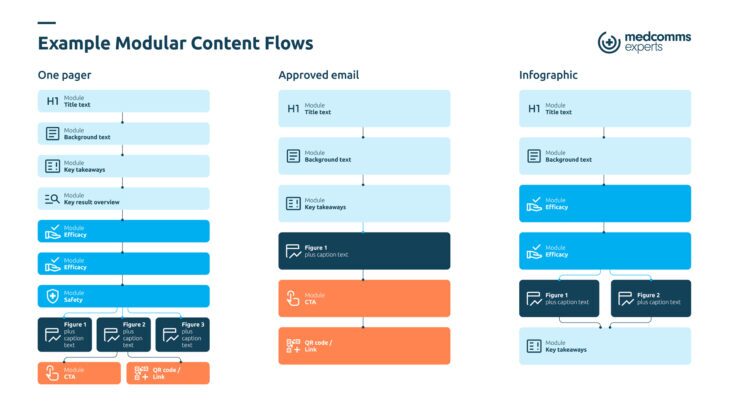
The Benefits of a Reusable Content Creation Framework
The framework supports a data-led strategy. You can better understand HCP needs, map deliverables, and identify areas where this saves time and enhances consistency. The lifecycle moves through component creation, regulatory approval, deployment across channels, performance tracking, and optimization.
Medical statements, graphs, and FAQs can be repurposed into emails, chatbot responses, slide decks, or HCP-facing content quickly while maintaining scientific accuracy. The framework supports both scale and personalization, and every audience receives appropriately tailored messaging while you maintain accuracy and consistency.
When reviewers encounter derivative content, it’s not their first exposure. While a full review remains necessary, they can move faster because the core messaging is already familiar. You reduce duplication without requiring a massive technology investment.
This kind of reusable framework requires clear documentation, designated ownership, team discipline, well-organized accessible storage (even SharePoint with proper governance), and regular review cycles.
What this doesn’t require? Perfect IT infrastructure, enterprise-wide software, automated updates, or impossible cross-functional coordination. In short, you gain many of the benefits of modular content without the barriers to implementation.
Setting Realistic Expectations for Content Development
A Centralized Content Creation Framework won’t transform your operations overnight. This is incremental improvement, not revolution.
You’ll gain clearer guidance on approved content, time savings through reduced duplication, better consistency without sacrificing flexibility, and smarter content delivered faster. You won’t gain automatic updates, perfect version control without manual oversight, complete elimination of review cycles, or fully integrated enterprise systems.
This approach reduces review bottlenecks. By pre-approving components before creating derivative materials, you shift the heaviest scientific validation to the front of the process. Subsequent reviews move faster because the compliance team has already vetted the core content—they’re confirming appropriate reuse, not re-evaluating science. The result: practical efficiency gains that work with your current systems and processes.
Don’t Wait for Perfect, Start With What’s Practical
Modular content has dominated Medical Affairs conference discussions for years. If it were achievable within pharmaceutical environments, someone would have implemented it by now. The industry is too complex, too regulated, and too varied for the enterprise-wide coordination that true modular content demands.
For Medical Affairs teams who are just starting out with the content creation framework approach, this is where you start. Identify your highest-value scientific content. Build a component repository around it. Get those components pre-approved. Give your teams structured guidance on strategic reuse.
Better still, partner with an agency that understands pharmaceutical realities and can design frameworks that function in your actual operating environment. You’ll see real benefits your teams need rather than reaching for theoretical ideals that require perfect infrastructure.